Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2CI2) at 25°C are 200 mm Hg and 41.5 mm Hg respectively. - Sarthaks eConnect | Largest Online Education Community

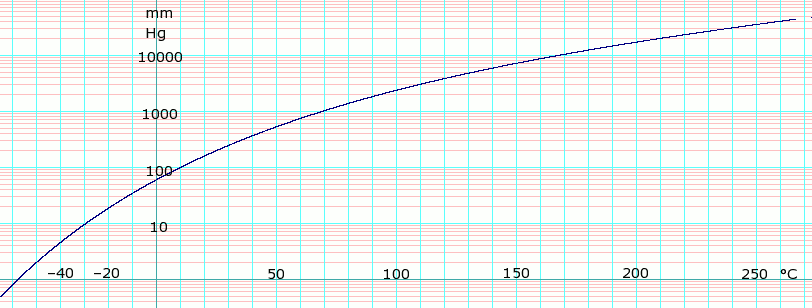
Temperature dependences (T, K) of the equilibrium vapour pressure (P,... | Download Scientific Diagram

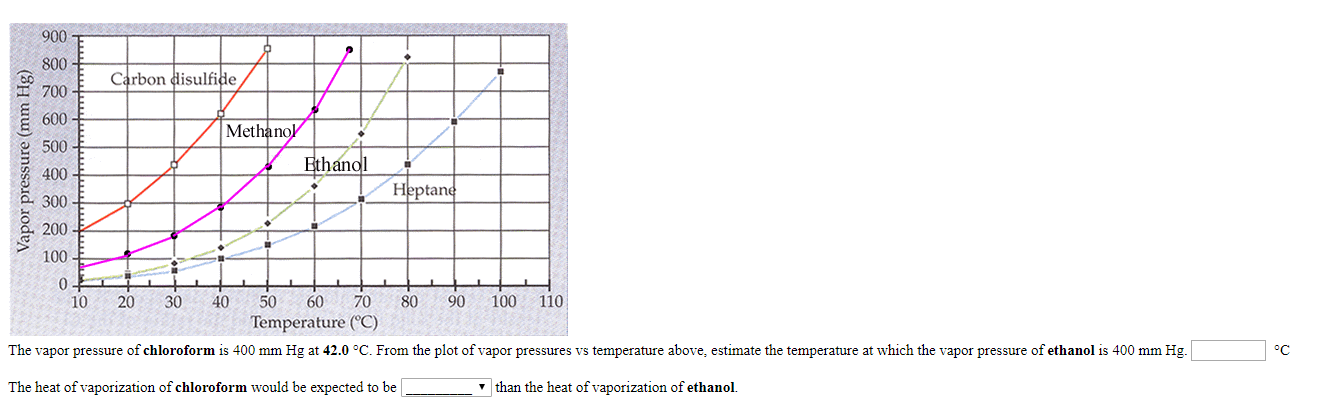
Use the following vapor pressure diagram to estimate the partial pressure of chloroform. | Homework.Study.com
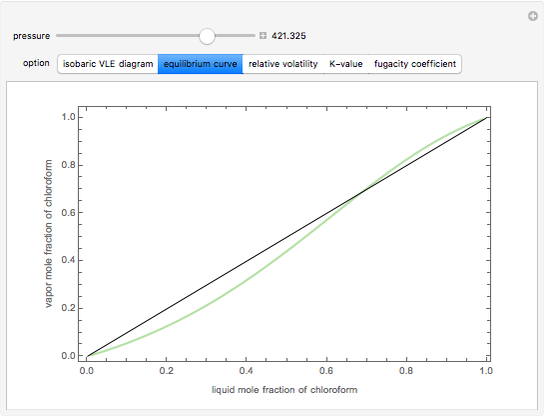
High Pressure Vapor-Liquid Equilibrium Data of a Binary Mixture of Chloroform and Acetone - Wolfram Demonstrations Project

Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 298 K are 200 mm Hg and 415 mm Hg respectively. Calculate the vapour pressure of the solution prepared by mixing 25.5 g

OneClass: Chloroform, CHCl3, has a vapor pressure of 197 mmHg at 23.0 °C, and its heat of vaporizati...

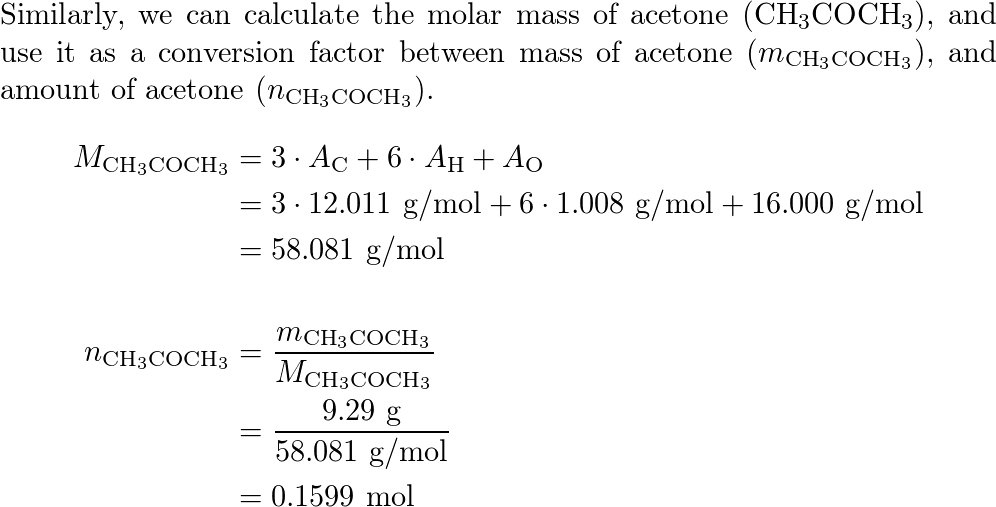
SOLVED: At a certain temperature the vapor pressure of pure chloroform CHCI;) is measured to be 0.74 atm Suppose solution is prepared by mixing 69.2 g of chloroform and 92.7 g of

Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 298K are 200mm Hg and 415mm Hg - YouTube
Review Questions: 1. An ethanol (e) and chloroform (c) liquid mixture contains a mole fraction of ethanol 0.20 x = . At 25 C°

Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform

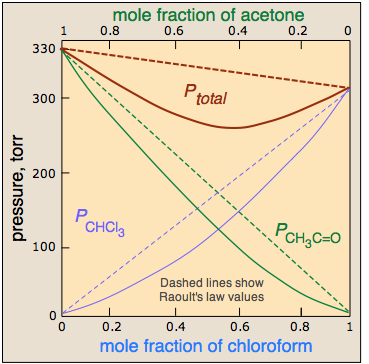
Vapour pressure of pure acetone and chloroform at 328 K are 741.8 mm Hg and 632.8 mm Hg respectively. Assuming that they form ideal solution over the entire range of composition, plot
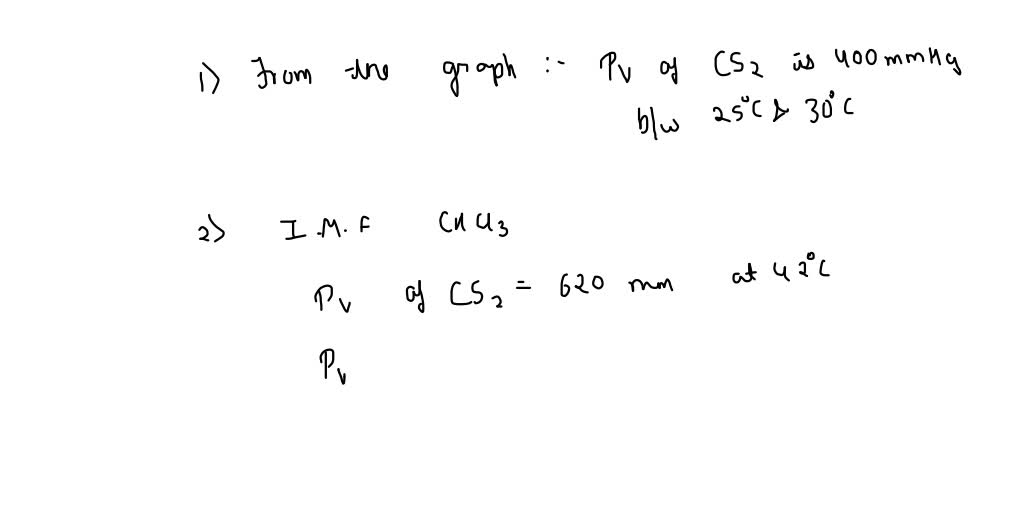
SOLVED: The vapor pressure of chloroform is 400 mm Hg at 42.0 °C. From the plot of vapor pressures vs temperature above, estimate the temperature at which the vapor pressure of ethanol
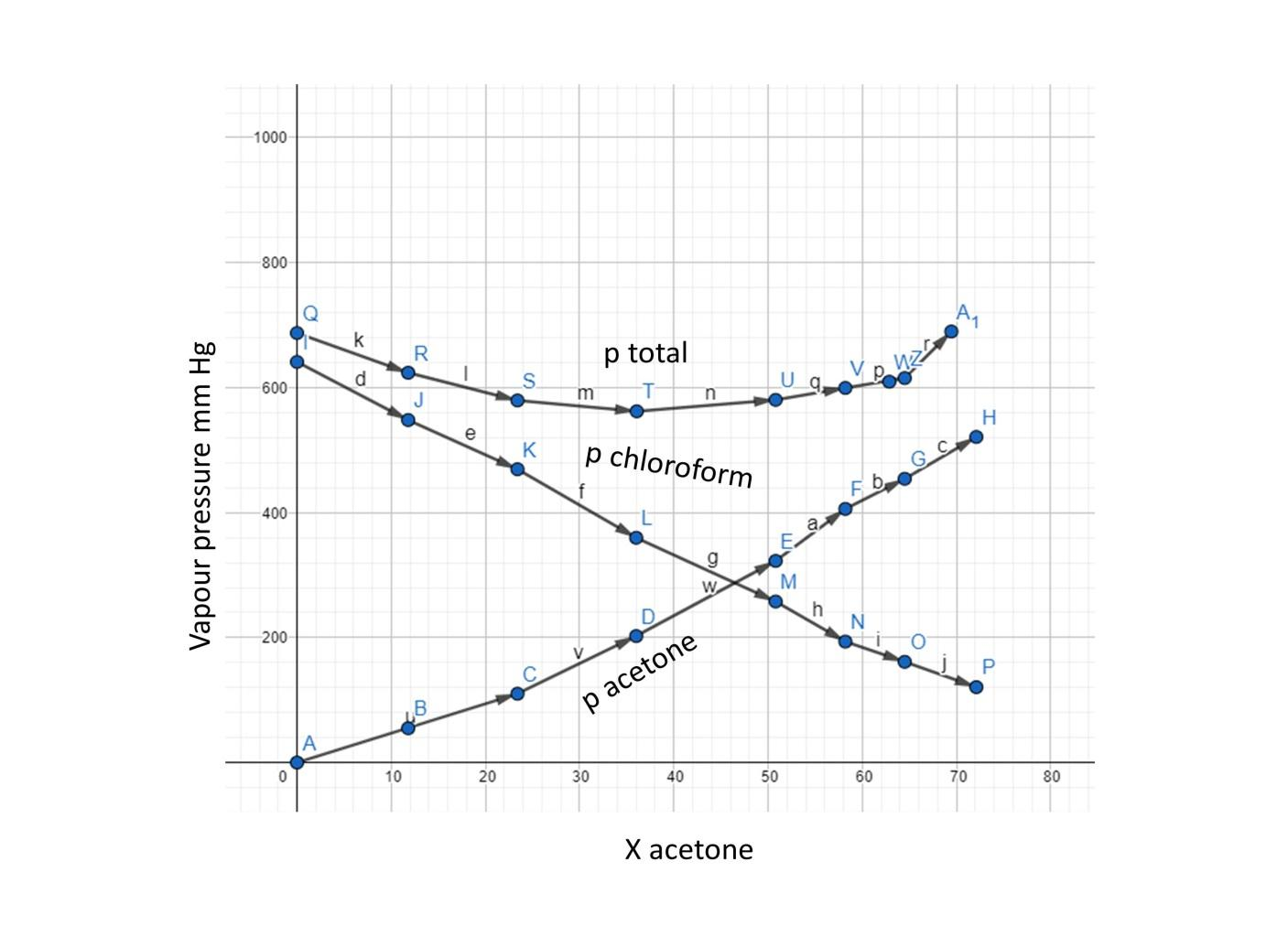
Vapour pressure of pure acetone and chloroform at $328\\,K$ are $741.8mmHg$ and $632.8mmHg$ respectively. Assuming that they form an ideal solution over the entire range of composition. Plot ${P_{total}},{P_{chloroform}}$ and ${P_{acetone}}$ as